A facile approach using MgCl2 to formulate high performance Mg2+ electrolytes for rechargeable Mg batteries†
Abstract
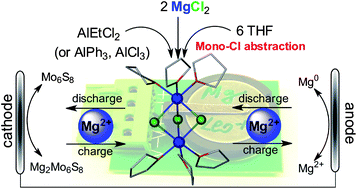
Rechargeable Mg batteries have been regarded as a viable battery technology for grid scale energy storage and transportation applications. However, the limited performance of Mg2+ electrolytes has been a primary technical hurdle to develop high energy density rechargeable Mg batteries. In this study, MgCl2 is demonstrated as a non-nucleophilic and cheap Mg2+ source in combination with Al Lewis acids (AlCl3, AlPh3 and AlEtCl2) to formulate a series of Mg2+ electrolytes, representing the simplest method to prepare Mg2+ conductive electrolytes (no precursor synthesis, free of recrystallization and giving quantitative yield). These electrolytes are characterized by high oxidation stability (up to 3.4 V vs. Mg), improved electrophile compatibility and electrochemical reversibility (up to 100% coulombic efficiency). Three electrolyte systems (MgCl2–AlCl3, MgCl2–AlPh3, and MgCl2–AlEtCl2) were fully characterized by multinuclear NMR (1H, 27Al{1H} and 25Mg{1H}) spectroscopies and electrochemical analysis. Single crystal X-ray diffraction and NMR studies consistently established molecular structures of the three electrolytes sharing a common Mg2+-dimer mono-cation, [(μ-Cl)3Mg2(THF)6]+, along with an anion (AlCl4−, AlPh3Cl− and AlEtCl3− respectively). Clean and dendrite free Mg bulk plating and viable battery performance were validated through representative studies using the MgCl2–AlEtCl2 electrolyte. The reaction mechanism of MgCl2 and the Al Lewis acids in THF is discussed to highlight the formation of the electrochemically active [(μ-Cl)3Mg2(THF)6]+ dimer mono-cation in these electrolytes and their improved performance compared to reported electrolytes using nucleophilic Mg2+ sources.

 Please wait while we load your content...
Please wait while we load your content...