Stabilization of selenium cathodes via in situ formation of protective solid electrolyte layer†
Abstract
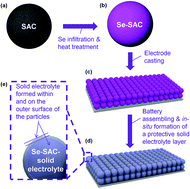
The lithium/selenium (Li/Se) rechargeable battery chemistry offers a higher energy density than traditional Li ion battery cells. However, high solubility of polyselenides in suitable electrolytes causes Se loss during electrochemical cycling, and leads to poor cycle stability. This study presents a simple technique to form a protective, solid electrolyte layer on the cathode surface. This protective layer remains permeable to Li ions, but prevents transport of polyselenides, thus dramatically enhancing cell cycle stability. The greatly reduced reactivity of polyselenides with fluorinated carbonates (such as fluoroethylene carbonates [FEC]) permits using their in situ reduction for low-cost formation of protective coatings on Se cathodes.

 Please wait while we load your content...
Please wait while we load your content...