Ionothermal synthesis of cobalt iron layered double hydroxides (LDHs) with expanded interlayer spacing as advanced electrochemical materials†
Abstract
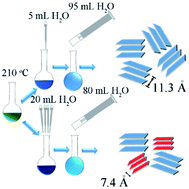
Traditional methods to synthesize layered double hydroxides (LDHs) with large interplanar spacing require the synthesis of precursor and subsequent ion exchange process. In this work, a one-step ionothermal strategy involving a “two-stage water injection” method is developed for the first time to synthesize cobalt iron layered double hydroxide (CoFe LDH) with 11.3 Å interplanar spacing from an easily available ionic liquid analog, deep eutectic solvent (DES) system. The expansion of interplanar spacing is accompanied with the occurrence of tetrahedrally coordinated cobalt, and the insertion of molecules possibly being acetaldehyde, ethanol and biuret. In addition, more CO32− and fewer Cl− and cyanate/isocyanate anions were intercalated. The nominal formula of a CoFe LDH sample with exclusively 11.3 Å (003) interplanar spacing was determined to be Co0.901Fe0.099C1.398O6.857N0.925H3.375Cl0.018. As a representative demonstration for its improved electrochemical activity, supercapacitor application and OER catalytic activity of the LDHs with different interplanar spacings are displayed, which indicates that the increasing interplanar spacing is a powerful way to improve the electrochemical activity of LDH. The innovative methodology developed in this work should be widely applicable based on the mechanism we investigated, thus providing a fast, simple and cheap way towards LDH with large interplanar spacings.

 Please wait while we load your content...
Please wait while we load your content...