Titanium carbide and carbonitride electrocatalyst supports: modifying Pt–Ti interface properties by electrochemical potential cycling†
Abstract
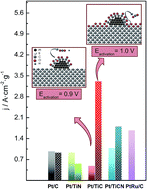
Titanium carbide and carbonitride are expected to be good materials to replace carbon as electrocatalyst supports, since they are chemically stable in acidic media and possess high electrical conductivity. However, they eventually can be transformed to titanium oxide, which is a thermodynamically stable compound, at potentials higher than 0.9 V (vs. RHE) in acidic media. In this communication, we report an enhanced catalytic activity towards CO and methanol electrooxidation on TiC and TiCN materials induced by surface oxides at the Pt/support interface. In particular, the current density obtained for Pt/TiC, activated up to 1.0 V, is 2-fold higher than that achieved with the commercial PtRu/C catalyst, which is accepted to be one of the best catalysts for methanol oxidation reaction.

 Please wait while we load your content...
Please wait while we load your content...