Defect induced sodium disorder and ionic conduction mechanism in Na1.82Mg1.09P2O7†
Abstract
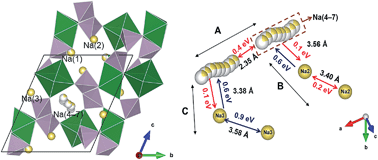
Phase pure sodium magnesium pyrophosphate Na1.82Mg1.09P2O7 was synthesized by a solid state reaction, and used as a model system to understand the Na+ dynamics in the related cathode materials Na2−xM1+x/2P2O7 (M = Fe, Mn, and Co). We found a three-dimensional framework structure formed by interconnecting Mg2O11 dimers with P2O7 pyrophosphate groups, where Na cages align along the [100] direction and are kinetically separated from each other. Diffuse distribution of Na was found inside the cage, and explained using a vacancy induced disorder model. Ionic conductivity is limited by inter-cage diffusion and measured to be 1.10 × 10−11 S cm−1 at room temperature with an activation energy of 0.77 eV. These features were further supported by bond-valence sum and lattice mechanics calculations.

 Please wait while we load your content...
Please wait while we load your content...