Single ion solid-state composite electrolytes with high electrochemical stability based on a poly(perfluoroalkylsulfonyl)-imide ionene polymer†
Abstract
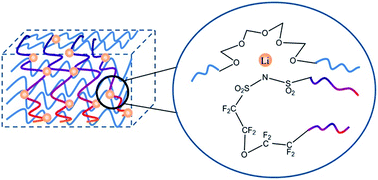
Composite single ion solid-state polymer electrolytes were prepared by blending lithiated poly(perfluoroalkylsulfonyl)imide (PFSILi) ionene with poly(ethylene oxide) (PEO) at various PFSILi contents. Their electrochemical performance was characterized by ionic conductivity, lithium ion transport number, cyclic voltammetry and galvanostatic cycling in batteries. The composite PEO–PFSI-25, containing 24.5% of PFSI, was found to have a maximum ionic conductivity of 1.76 × 10−4 S cm−1 at 80 °C. Because of the immobilization of the anionic perfluorosulfonimide groups on the backbone of the ionene polymer with flexible and stable perfluoroether connectors, the composite had unusually high electrochemical stability (up to 5.5 V vs. Li+/Li) and a low anion transference number. The PFSILi unit interacts strongly with the PEO segments, resulting in the depression of PEO crystallization and the formation of multi-dimensional ionic crosslinks within the composite to impart dimensional and cycling stability to the electrolyte system. LiFePO4/Li battery prototypes using PEO–PFSILi polymer electrolytes, with reversible capacity of 140 mA h g−1 at 80 °C and 0.2 °C, were able to maintain 50 stable cycles for 1000 h at 70 °C and 0.1 °C.

 Please wait while we load your content...
Please wait while we load your content...