Multiscale structure of calcium- and magnesium-silicate-hydrate gels†
Abstract
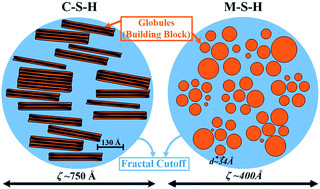
Concrete is the world's most widely used building material. However, the production of CaO-based cements generates large amounts of anthropogenic emissions of CO2. Among different strategies to reduce CO2 emissions, newly developed MgO-based cements, though currently suffering from inferior mechanical properties, are some of the most promising and attractive options. By combining wide- and small-angle X-ray scattering and electron microscopy, we identified differences in the multiscale structure of the two main binding phases: the calcium-silicate-hydrate (C-S-H) gel for CaO-based cements and the magnesium-silicate-hydrate (M-S-H) gel for MgO-based cements. We found the primary unit at the nanoscale level of C-S-H to be a multilayer disk-like globule, whereas for M-S-H it is a spherical globule. These prominent differences result in diverse microstructures, leading to disparities in mechanical properties and durability for the associated cements. Modulating the M-S-H structure and enhancing the compatibility between C-S-H and M-S-H will be the key to improve the robustness of eco-friendly MgO-based binders.

 Please wait while we load your content...
Please wait while we load your content...