4,4′-Biphenyldicarboxylate sodium coordination compounds as anodes for Na-ion batteries†
Abstract
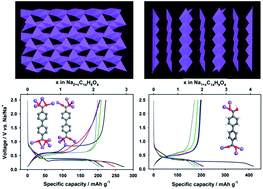
Novel 4,4′-biphenyldicarboxylate (bpdc) sodium salts with different compositions were evaluated for the first time as anodes for Na-ion batteries, and their crystal structures and corresponding electrochemical performances were analyzed. The structure of the bpdc-sodium salts was modified using precipitation and solvothermal methods to afford three different crystal structures with different degrees of deprotonation of the carboxylic acid (COOH) groups and different coordination of the water molecule, as determined by single crystal X-ray diffraction. The extent of deprotonation in bpdc-sodium salts not only affects their electrochemical performance, but also affects the corresponding reaction mechanisms. The fully deprotonated bpdc-disodium salt exhibits a promising electrochemical performance with a reversible capacity of about 200 mA h g−1 at ca. 0.5 V vs. Na/Na+, stable cycle performance over 150 cycles, and an excellent rate performance of 100 mA h g−1 even at a 20 C rate, which are better than those of the partially deprotonated bpdc-sodium salt. The sodiation–desodiation of bpdc-sodium salts proceeds in a two-phase reaction, regardless of the degree of deprotonation. However, unlike the fully deprotonated bpdc-disodium salt, which shows a reversible phase transition during sodiation and desodiation, the partially deprotonated bpdc-sodium salt exhibits an irreversible phase transition during cycling.

 Please wait while we load your content...
Please wait while we load your content...