Enhanced CO2 absorption kinetics in lithium silicate platelets synthesized by a sol–gel approach
Abstract
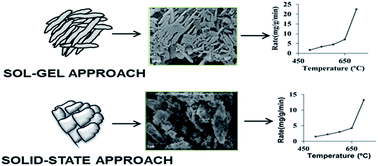
Platelet-shaped lithium orthosilicate particles synthesized by a sol–gel approach employing the precursors lithium nitrate and colloidal silica displayed enhanced absorption kinetics for CO2 compared to the powders prepared by a solid-state reaction process involving Li2CO3 and silica. The sol–gel samples showed a CO2 absorption capacity of 350 mg g−1 at an absorption rate of 22.5 mg g−1 min−1, a value 70% higher than the rate of 13.2 mg g−1 min−1 measured with the solid-state samples under similar conditions. The higher sorption kinetics of CO2 by the sol–gel derived lithium orthosilicate could be attributed to the unique platelet morphology of the particles, which have a very small thickness. A porous carbon mesh coated with the sol–gel based particles exhibited CO2 absorption capacity of 150 mg g−1 at an absorption rate of 37.5 mg g−1 min−1. This supported absorbent also showed stable absorption and desorption performance for the 8 cycles examined in this study. The excellent absorption characteristics of the sol–gel prepared powders, more specifically the coated strips, provide a successful pathway for the commercialisation of these materials.

 Please wait while we load your content...
Please wait while we load your content...