MoO2 nanobelts@nitrogen self-doped MoS2 nanosheets as effective electrocatalysts for hydrogen evolution reaction†
Abstract
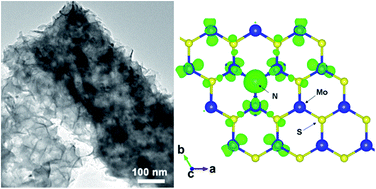
Advanced materials for electrocatalytic water splitting are central to renewable energy research. In this study, MoO2 nanobelts@nitrogen self-doped MoS2 nanosheets are produced by nitridation and sulfuration treatments of MoO3 nanobelts. The material structures are characterized by a variety of techniques including scanning electron microscopy, transmission electron microscopy, Raman scattering, X-ray photoelectron spectroscopy, and X-ray diffraction spectroscopy. It is found that because of nitrogen doping and the abundance of exposed active edges, the heterostructures exhibit high electronic conductivity, and more importantly, enhanced and stable electrocatalytic activity in hydrogen evolution reaction (HER), as manifested in electrochemical studies. The onset potential is found to be only −156 mV (vs. RHE), which is 105 mV more positive than that of pure MoS2 under identical experimental conditions. The corresponding Tafel slope is estimated to be 47.5 mV dec−1, even slightly less than that of commercial 10 wt% Pt/C (49.8 mV dec−1), suggesting that the reaction dynamics is largely determined by the electrochemical desorption of hydrogen. This is accounted for by nitrogen doping that leads to an enhanced electronic conductivity of the heterostructures as well as a high density of spinning electron states around the N and Mo atoms in MoS2 nanosheets that are the active sites for HER, as manifested in density functional theory studies of a N-doped MoS2 monolayer.

 Please wait while we load your content...
Please wait while we load your content...