Sorption interactions of plutonium and europium with ordered mesoporous carbon†
Abstract
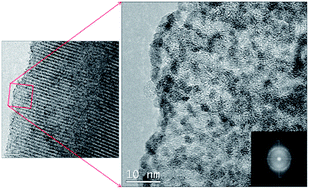
Both 3d-cubic FDU-16-type and 2d-hexagonal C-CS-type ordered mesoporous carbons (OMCs) were synthesized to test their application as radionuclide sorbent materials. A portion of each OMC was oxidized with acidic ammonium persulfate (APS), and the physicochemical properties of all four OMCs were characterized with several techniques. Based on plutonium (Pu) sorption and desorption tests with FDU-16, oxidized FDU-16-COOH, C-CS, and oxidized C-CS-COOH, the C-CS-COOH was the most effective OMC for sorption of Pu over a wide pH range. Batch sorption interactions of C-CS and C-CS-COOH were further explored with Pu(VI) and Eu(III) to determine the uptake capacities, sorption kinetics, and effects of ionic strength. The nature of the Pu sorption reaction was also probed via X-ray absorption spectroscopy (XAS) and transmission electron microscopy (TEM). The highly oxidized surface, large pores, and high surface area of C-CS-COOH make it a very effective general scavenger for actinide and lanthanide cations. Pu and Eu uptake by C-CS-COOH appears to be dictated by chemisorption, and the Langmuir Eu capacity (138 mg g−1 from pH 4 solution) is higher than those previously reported for many other adsorbents. Pristine C-CS has a low affinity for Eu(III), but is an excellent sorbent of PuO2 nanocrystals (∼3 nm diameter), which are formed because the carbon reduces Pu(VI) and Pu(V) to Pu(IV). Plutonium is also reduced by C-CS-COOH, but PuO2 colloid formation in pH 4 solution is prevented by carboxyl complexation of Pu(IV) at the C-CS-COOH surface.

 Please wait while we load your content...
Please wait while we load your content...