3D Co3O4 and CoO@C wall arrays: morphology control, formation mechanism, and lithium-storage properties†
Abstract
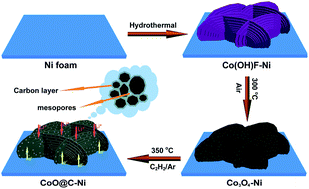
In this work, a rational hydrothermal route is designed to prepare Co(OH)F wall arrays assembled with ultrathin nanosheets on conductive nickel foam (denoted as Co(OH)F–Ni) with robust adhesion properties. Ammonium fluoride is utilized to organize single-crystalline nanosheets into wall arrays constructed from ultrathin nanosheets in this method. The morphological evolution process of this organized product has been investigated by examining different reaction intermediates in detail. A novel growth mechanism, a gradual crystallization self-assembly process via Ostwald ripening, is explored for the first time to understand the formation of wall arrays assembled with nanosheets. Subsequently, mesoporous Co3O4 wall arrays are fabricated via thermal decomposition, maintaining the original morphology of precursor Co(OH)F. Through the next step of chemical vapor deposition (CVD) in an atmosphere of acetylene, carbon has been integrated to form carbon-coated CoO wall arrays on nickel foam (denoted as CoO@C–Ni). Intriguingly, the present CVD process can also produce onion-like graphitic carbon at relatively low temperatures (300–350 °C) near the substrate because of the catalysis of nickel foam. 3D self-supported Co3O4 and CoO@C electrodes allow direct and close contact of electroactive materials and the current collector, greatly improving the system's conductivity and structural stability. Furthermore, the self-supported electrodes consist of wall arrays grown directly on current-collecting substrates, and effectively simplify the preparation process of electrodes without any requirement of other ancillary materials, such as conductive additives or binders. Each wall is fixed to the current collector, resulting in fast charge transfer. Benefitting from the unique structural features, the as-prepared cobalt oxide-based ordered hierarchically porous 3D electrodes, especially CoO@C wall arrays, manifest excellent electrochemical performance with outstanding rate capability and good cycling stability for highly reversible lithium storage.

 Please wait while we load your content...
Please wait while we load your content...