Controllable synthesis of high-performance LiMnPO4 nanocrystals by a facile one-spot solvothermal process†
Abstract
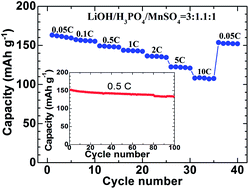
Olivine-type LiMnPO4 has been considered as a promising cathode material for the next-generation high-power lithium ion batteries. Preparing high-performance LiMnPO4 cathodes by a simple approach has been a subject of much scientific inquiry for several years. Here, we report a simple solvothermal synthesis of LiMnPO4 nanocrystals using LiOH·H2O, H3PO4 and MnSO4·H2O as the precursors and ethylene glycol as the reaction medium. We found that the ratio of the starting materials exerts a significant influence on the morphology, size and crystal orientation of LiMnPO4 nanocrystals. We confirmed the critical role of H+ concentration in altering the crystallization characteristics of LiMnPO4. The results showed that after carbon coating, the plate-like LiMnPO4, which was synthesized from the precursor with a LiOH/H3PO4/MnSO4 ratio of 3 : 1.1 : 1, exhibited the best electrochemical performance, yielding a discharge capacity of 108.2 mA h g−1 at 10 C and maintaining a discharge capacity of 133.5 mA h g−1 after 100 cycles at 0.5 C. This simple, one-spot solvothermal preparation method sheds light on the synthesis of high-performance LiMnPO4 cathode material.

 Please wait while we load your content...
Please wait while we load your content...