A facile preparation method of surface patterned polymer electrolyte membranes for fuel cell applications†
Abstract
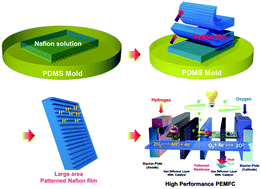
We report a facile patterning method that facilitates production of large-area platforms with well-arrayed micro/nanopatterns of a polymer electrolyte membrane (PEM) at low cost using an elastomeric mold at room temperature without hot-pressing. Membrane–electrode interfacial properties on the cathode side are controlled by the patterned structure of the membrane, which in turn directly affects the electrochemically active surface area (ECSA) and Pt utilization of the catalyst. This confirmed that electrochemical properties improve the performance of the membrane electrode assembly (MEA). A MEA fabricated with a 3 × 5 μm (width × gap) micropatterned Nafion membrane exhibits a current density of 1.79 A cm−2 at 0.6 V and a power density of 1.26 W cm−2 at 75 °C; these values are 53% and 59% greater than those of the corresponding MEA without a patterned membrane, respectively, and are among the highest performances reported for polymer electrolyte membrane fuel cells (PEMFCs). However, use of a nanopatterned membrane decreases the performance due to insufficient infiltration of the ionomer into the grooved surface, leading to a poor mechanical/electrical contact between the membrane and the electrode. Membrane morphology and the structure of the membrane–electrode interface are characterized by field emission scanning electron microscopy (FE-SEM), cyclic voltammetry (CV), and impedance spectroscopy.

 Please wait while we load your content...
Please wait while we load your content...