Synthesis of hydrogenated TiO2–reduced-graphene oxide nanocomposites and their application in high rate lithium ion batteries
Abstract
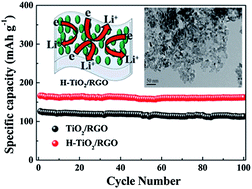
A hydrogenated TiO2–reduced-graphene oxide (H-TiO2–RGO) nanocomposite is synthesised via a facile one-pot hydrogenation treatment process. The morphologies and structures are characterized by transmission electron microscopy (TEM) and X-ray diffraction (XRD). The nitrogen adsorption–desorption isotherms revealed that the H-TiO2–RGO exhibited large specific surface area of 114.4 m2 g−1. Compared with the TiO2–RGO nanocomposite, the H-TiO2–RGO nanocomposite exhibits a much higher rate capability and better capacity retention. At a current rate of 5 C, the reversible capacity of the H-TiO2–RGO electrode is up to 166.3 mA h g−1 and with only 2.4% capacity loss after 100 cycles. The excellent electrochemical performance is strongly related to the high electronic conductivity derived from hydrogenated TiO2 frameworks and the good contact between the zero-dimensional (0D) H-TiO2 nanoparticles with two-dimensional (2D) reduced-graphene oxide nanosheets, which efficiently shortened the Li+ diffusion path lengths, enhanced the electrolyte–active material contact area and facilitated rapid e− transfer.

 Please wait while we load your content...
Please wait while we load your content...