Solvent-free synthesis and stability of MgB12H12†
Abstract
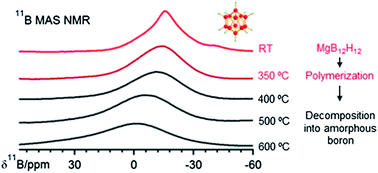
MgB12H12 has been widely discussed as an intermediate in the hydrogen sorption cycles of Mg(BH4)2, but its properties such as stability and reactivity are still unknown. We achieved the synthesis of MgB12H12via the reaction between Mg(BH4)2 and B2H6 at 100 to 150 °C. When bulk Mg(BH4)2 was used as the starting material, a yield of 10.2 to 22.3 mol% was obtained, which was improved to 92.5 mol% by using Mg(BH4)2 nanoparticles. The as-synthesized MgB12H12 decomposed into boron between 400 and 600 °C, preceded by a possible polymerization process. The formation mechanism of MgB12H12 and its role in the decomposition process of Mg(BH4)2 are discussed.

 Please wait while we load your content...
Please wait while we load your content...