Enhanced electrochemical performance of novel K-doped Co3O4 as the anode material for secondary lithium-ion batteries
Abstract
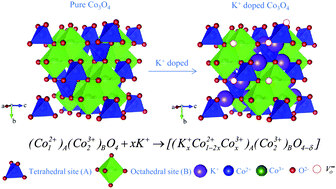
K-doped Co3O4 was prepared by a solvothermal method in polyol medium, followed by annealing at a low temperature of 400 °C for 5 h. The obtained samples were characterized by the synchrotron X-ray diffraction pattern, field-emission scanning electron microscopy, energy-dispersive X-ray spectroscopy, field-emission transmission electron microscopy and high-resolution transmission electron microscopy. Synchrotron XRD analysis demonstrates that the K+ ion doping caused no change in the phase structure, and a highly crystalline KxCo3−xO4−δ (x = 0.08) powder without any impurity was obtained. When applied as the anode material, the K+-doped Co3O4 electrode exhibits a much better rate capability and cycling stability, and could retain a charge capacity of 351.3 mA h g−1 at 3 C, while undoped Co3O4 exhibits only 144.3 mA h g−1 at the same rate. In addition, the electrochemical impedance spectroscopy also reveals that the K+-doped Co3O4 electrode has the highest electronic conductivity compared to an undoped sample. However, the improvement in the doped sample is due to the influence of K+ ions on the increased electronic conductivity, diffusion efficiency, and kinetic properties of Co3O4 during the lithiation and delithiation process. This material shows promising potential for use in high-rate anodes for lithium-ion batteries.

 Please wait while we load your content...
Please wait while we load your content...