De novo assembly of a mesoporous beta zeolite with intracrystalline channels and its catalytic performance for biodiesel production†
Abstract
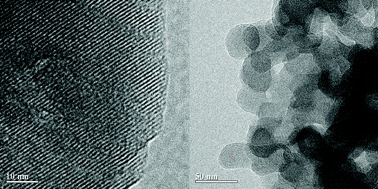
A mesoporous beta zeolite was hydrothermally prepared directly by silanizing silica without any mesoporous template via the bond-blocking principle. Si–C bond-blocking arose during the crystalline growth. The crystallization took more than 10 days, but the material had a fairly stable structure and could even be processed up to 32 days later in the hydrothermal system. XRD, N2-adsorption/desorption and TEM/SEM characterization of the materials indicated that the beta zeolite is truly a sponge-like mesoporous zeolite with a BEA topological structure, which consists of self-sustaining macroscopic sized zeolitic aggregates assembled from nanosized crystalline domains of beta zeolite with intracrystalline mesopores. The mesoporous beta zeolite possessed an extremely large external surface area and adjustable mesoporosity. Compared to conventional beta zeolite, FTIR results of pyridine (Py) and 2,6-di-tert-butylpyridine (DTBPy) demonstrated an increase of the Lewis-site contribution and a large improvement for the accessibility of bulky molecules in the mesoporous beta zeolite. Finally the mesoporous beta zeolite exhibited significant activity in the transesterification reaction of triolein to afford methyl oleate (biodiesel) due to the accessibility increase and diffusion-limitation reduction of large lipids to acid sites in the H-beta zeolite framework.

 Please wait while we load your content...
Please wait while we load your content...