Electrochemical performance of Na/NaFePO4 sodium-ion batteries with ionic liquid electrolytes†
Abstract
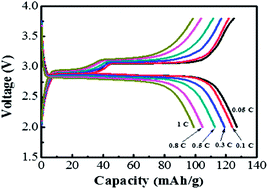
Rechargeable Na/NaFePO4 cells with a sodium bis(trifluoromethanesulfonyl)imide (NaTFSI)-incorporated butylmethylpyrrolidinium (BMP)–TFSI ionic liquid (IL) electrolyte are demonstrated with an operation voltage of ∼3 V. High-performance NaFePO4 cathode powder with an olivine crystal structure is prepared by chemical delithiation of LiFePO4 powder followed by electrochemical sodiation of FePO4. This IL electrolyte shows high thermal stability (>400 °C) and non-flammability, and is thus ideal for high-safety applications. The effects of NaTFSI concentration (0.1–1.0 M) on cell performance at 25 °C and 50 °C are studied. At 50 °C, an optimal capacity of 125 mA h g−1 (at 0.05 C) is found for NaFePO4 in a 0.5 M NaTFSI-incorporated IL electrolyte; moreover, 65% of this capacity can be retained when the charge–discharge rate increases to 1 C. This ratio (reflecting the rate capability) is higher than that found in a traditional organic electrolyte. With a 1 M NaTFSI-incorporated IL electrolyte, a 13% cell capacity loss after 100 charge–discharge cycles is measured at 50 °C, compared to the 38% observed in an organic electrolyte under the same conditions.

 Please wait while we load your content...
Please wait while we load your content...