Electrochemical extraction of Ti5Si3 silicide from multicomponent Ti/Si-containing metal oxide compounds in molten salt
Abstract
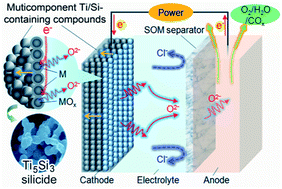
Ti5Si3 silicide has been extracted directly from complex multicomponent Ti/Si-containing metal oxide compounds by electro-deoxidation in molten calcium chloride using an inert solid oxide oxygen-ion-conducting membrane (SOM) based anode. Studies on the microstructure evolution and electrochemical extraction mechanism show that the formation of Ti5Si3 and the removal of impurity elements happened simultaneously during the electro-deoxidation process. It is found that the electro-deoxidation generated Ti5Si3 micro-particles typically possess a smooth surface, which could contribute to create a continuous anti-oxidation surface layer with excellent high-temperature oxidation resistance property. Consideration is also given to the parameters of electrolysis and the electrochemical characteristics including chemical and/or electrochemical reactions during the electro-deoxidation process, and then a relevant kinetic model is proposed.

 Please wait while we load your content...
Please wait while we load your content...