AB–MH (Ammonia Borane–Metal Hydride) composites: systematic understanding of dehydrogenation properties
Abstract
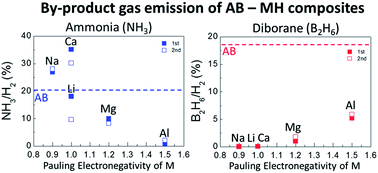
Dehydrogenation properties of AB–MH (Ammonia Borane–Metal Hydride, M = K, Na, Li, Ca, Mg, Al) composites were systematically investigated by thermal and mass analyses. The results suggest that the Pauling electronegativity of M, χp, is a good indicator to predict the phases of composites, the dehydrogenation temperature and the amount of by-product gases (NH3 and B2H6). The phases of composites were classified by χp as follows. MBH4 was formed for M = K, Na (χp ≤ 0.9), MNH2BH3 was formed for M = Na, Li (0.9 ≤ χp ≤ 1.0) and no new compounds were formed for M = Ca, Mg, Al (1.0 ≤ χp). The 1st dehydrogenation temperatures of the samples (M = Na, Li, Ca, Mg) were 10–20 °C lower than that of AB itself (χp ≤ 1.2). The amount of NH3 was decreased as χp increased. On the other hand, the amount of B2H6 was decreased as χp decreased. The emission of B3H6N3 could occur by the reaction of NH3 and B2H6. Finally, AB–MAlH4 (M = Na, Li) composites, which were prepared based on the indicator, showed superior potential as hydrogen storage materials because they did not desorb any by-products NH3, B2H6 and B3H6N3.

 Please wait while we load your content...
Please wait while we load your content...