Ethanol-assisted hydrothermal synthesis of LiNi0.5Mn1.5O4 with excellent long-term cyclability at high rate for lithium-ion batteries†
Abstract
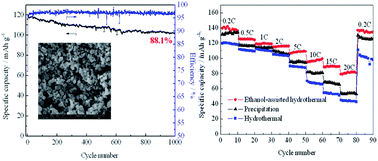
High voltage spinel LiNi0.5Mn1.5O4 has been synthesized by an ethanol-assisted hydrothermal method. LiNi0.5Mn1.5O4 has also been synthesized by a precipitation method and hydrothermal method for comparison. The materials were characterized by X-ray diffraction, Raman spectroscopy, scanning electron microscopy, X-ray photoelectron spectroscopy and electrochemical tests. The ethanol-assisted hydrothermal process improves the dispersity and decreases the size of particles in the presence of ethanol. With small size particles, LiNi0.5Mn1.5O4 has an excellent rate capability. Its discharge capacity is 81.7 mA h g−1 at a high rate of 20 C. On the other hand, the ethanol-assisted hydrothermal process mixes the reagents homogeneously and improves the crystallinity. It leads to low impurities and low Mn3+ ion content, which are beneficial for electrochemical performance. The LiNi0.5Mn1.5O4 exhibits remarkable long-term cyclability. After 1000 cycles at a 5 C discharge rate, its discharge capacity is 102.1 mA h g−1 with a capacity retention ratio of 88.1%. It also has good high temperature performance with a capacity retention of 82.0% after 200 cycles at 55 °C.

 Please wait while we load your content...
Please wait while we load your content...