Electrochemical substitution of sodium ions with protons in phosphate glass to fabricate pure proton conducting glass at intermediate temperatures
Abstract
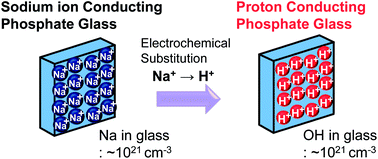
Electrochemical substitution involving electrochemical oxidation of hydrogen and proton injection into oxide glass accompanied by electrochemical reduction of alkali ions and discharge of metallic alkali out of the glass has recently been proposed as a proton injection technique. Herein, this electrochemical substitution technique was applied to phosphate glass with a composition of WO3–35NaO1/2–8NbO5/2–5LaO3/2–51PO5/2 (1W-glass). Temporal evolution of the substitution of sodium ions with protons was studied using a range of techniques. The concentration depth profiles of sodium ions and protons were mirror images of each other and the amount of injected protons quantitatively agreed with that of the decrease of sodium ions. The concentration of W5+ ions formed after substitution was only 3 ppm of the amount of injected protons, so reduction of W6+ ions in glass is not essential for proton injection. Raman spectra of the glasses indicated that the glass network structure did not change during electrochemical substitution; therefore, the protons substitute sodium ions not only quantitatively but also structurally. The glass after substitution attained a high proton concentration of 4.6–6.6 × 1021 cm−3 and pure proton conduction with a conductivity of 4.0 × 10−4 S cm−1 at 250 °C. A test fuel cell using electrochemically substituted 1W-glass as a solid electrolyte generated a maximum power density of 0.35 mW cm−2 operating at 250 °C.

 Please wait while we load your content...
Please wait while we load your content...