Mussel-inspired nitrogen-doped graphene nanosheet supported manganese oxide nanowires as highly efficient electrocatalysts for oxygen reduction reaction†
Abstract
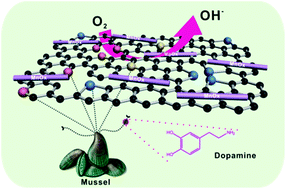
Electrocatalysts for oxygen reduction reaction (ORR) play a vital role in determining the performance of fuel cells and metal–air batteries. Carbon nanomaterials doped with heteroatoms are highly attractive by virtue of their excellent electrocatalytic activity, high conductivity and large surface area. This study reports the synthesis of a highly efficient electrocatalyst based on nitrogen-doped (N-doped) graphene nanosheets (NG) using mussel-inspired dopamine as a nitrogen source. Dopamine undergoes oxidative polymerization that can functionalize the surface of graphene and also introduces nitrogen atoms onto the graphene nanosheets upon pyrolysis. N-doping not only leads to improved catalytic activity, but it also provides anchoring sites for the growth of electroactive amorphous manganese oxide nanowires on the graphene nanosheets (NG/MnOx). On the basis of a Koutecky–Levich plot, it is found that the hybrid NG/MnOx catalyst exhibits excellent catalytic activity with a direct four-electron pathway in ORR. Furthermore, the hybrid electrocatalyst possesses superior stability and gives a low yield of peroxide compared to commercial Pt/C catalysts. This suggests that the unique combination of an N-doped graphene support and amorphous MnOx nanowires can synergistically improve the catalytic activity for ORR.
- This article is part of the themed collection: Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...