Template-free synthesis of high surface area nitrogen-rich carbon microporous spheres and their hydrogen uptake capacity†
Abstract
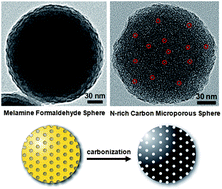
Nitrogen-rich carbon microporous spheres (NCMSs) are synthesized for the first time by carbonizing melamine-formaldehyde spheres (MFSs). The surface area of NCMSs is increased approximately 100 times after the carbonization process. The NCMS synthetic process is facile and convenient to reproduce since the hard template production step is eliminated. In addition, the 13C MAS NMR spectrum for the NCMSs shows that the spheres are consisting of carbon atoms with multiple sp2 bonding configurations. Moreover, we find that nanopores in NCMSs offer high uptake capacity for hydrogen molecules.

 Please wait while we load your content...
Please wait while we load your content...