In situ synthesis of well crystallized rhodium sulfide/carbon composite nanospheres as catalyst for hydrochloric acid electrolysis†
Abstract
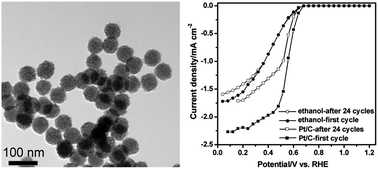
Rhodium sulfide/carbon nanocomposites were synthesized via a one-step alcohol-thermal method at 400 °C from Rh6(CO)16 and elemental S. Characterizations by X-ray diffraction, scanning electron microscopy, transmission electron microscopy, Raman spectroscopy and X-ray photoelectron spectroscopy revealed that the products were composed of amorphous carbon with spherical morphology and numerous highly crystallized Rh2S3/Rh17S15 nanocrystals, presented as uniform size composite nanospheres of 45–90 nm diameter. The crystallographic composition of rhodium sulfide in those composites depended on the initial S/Rh molar ratio and utilized solvent. Such hybrid nanomaterials displayed good dispersion of the rhodium sulfide nanoparticles, high surface area and extraordinary thermal/chemical resistance, making them attractive materials for electrocatalytic application of HCl electrolysis. Cyclic voltammetry and rotating disk electrode measurements were employed to evaluate the catalytic performance for oxygen reduction reaction in HCl electrolysis. It was illustrated that all rhodium sulfide/carbon nanocomposites were active towards oxygen reduction reaction. Especially, the catalyst synthesized in ethanol containing Rh17S15 phase outperformed commercial Pt/C in stability.

 Please wait while we load your content...
Please wait while we load your content...