Porous chromium-based ceramic monoliths: oxides (Cr2O3), nitrides (CrN), and carbides (Cr3C2)†
Abstract
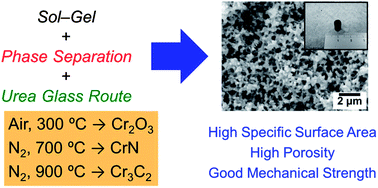
In this research, we have successfully prepared hierarchically porous chromium-based non-oxide ceramic monoliths (chromium nitride (CrN) and chromium carbide (Cr3C2)) by facile two-step methods. By utilizing an epoxide-mediated sol–gel reaction accompanied by phase separation, a well-defined macroporous structure is tailored in the chromium (oxy)hydroxide–urea composites in the presence of poly(acrylic acid). Urea works as a nitrogen and/or carbon source through the following heat-treatment, whereas poly(acrylic acid) behaves as a phase separation inducer as well as a network-supporting constituent. The macroporous morphology of xerogels is controllable by changing the starting composition. Heat-treatment for thus obtained xerogels under an air atmosphere provides chromium oxide (Cr2O3), whereas that under a nitrogen atmosphere leads to the crystallization of CrN at 700 °C and Cr3C2 at 900 °C without any deterioration of the porous morphologies. The heat-treated samples possess a high specific surface area up to 450 m2 g−1 as well as high porosity over 80%, preserving good mechanical strength. This facile and novel synthesis strategy has a potential extendability to other macroporous monoliths with high surface area based on various metal nitrides and carbides, which are useful in various applications such as catalysts, semiconductor devices, magnetic materials, and refractory materials.

 Please wait while we load your content...
Please wait while we load your content...