Chiral/ring closed vs. achiral/open chain triazine-based organogelators: induction and amplification of supramolecular chirality in organic gels†
Abstract
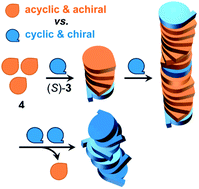
The purpose of this study is to compare the gelling behavior of two molecules: a chiral compound and its achiral counterpart. The chiral partner is characterized by a rigid, chiral pyrrolidine nucleus, while the achiral one contains a flexible diethanolamine moiety. The chiral compound is an already known good organogelator, but also the achiral compound shows remarkable gelling properties. Very interestingly, a small fraction of the chiral compound induces chirality and strong CD effects in its aggregates with the achiral one. The observed chirality amplification corresponds to a peculiar sergeant-and-soldier effect. Molecular modelling and CD calculations suggested a model for the supramolecular assembly of hetero-aggregates that fits the experimental data.

 Please wait while we load your content...
Please wait while we load your content...