Equilibrium and non-equilibrium cluster phases in colloids with competing interactions
Abstract
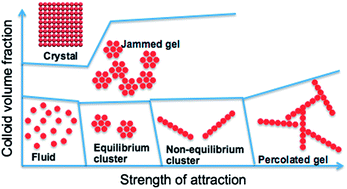
The phase behavior of colloids that interact via competing interactions – short-range attraction and long-range repulsion – is studied by computer simulation. In particular, for a fixed strength and range of repulsion, the effect of the strength of an attractive interaction (ε) on the phase behavior is investigated at various colloid densities (ρ). A thermodynamically stable equilibrium colloidal cluster phase, consisting of compact crystalline clusters, is found below the fluid–solid coexistence line in the ε–ρ parameter space. The mean cluster size is found to linearly increase with the colloid density. At large ε and low densities, and at small ε and high densities, a non-equilibrium cluster phase, consisting of elongated Bernal spiral-like clusters, is observed. Although gelation can be induced either by increasing ε at constant density or vice versa, the gelation mechanism is different in either route. While in the ρ route gelation occurs via a glass transition of compact clusters, gelation in the ε route is characterized by percolation of elongated clusters. This study both provides the location of equilibrium and non-equilibrium cluster phases with respect to the fluid–solid coexistence, and reveals the dependencies of the gelation mechanism on the preparation route.

 Please wait while we load your content...
Please wait while we load your content...