Correlation between the geometrical shape and growth behaviour of surfactant micelles investigated with small-angle neutron scattering†
Abstract
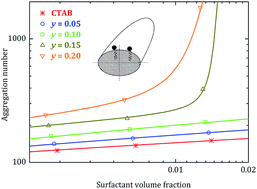
The correlation between the growth behaviour and geometrical shape for CTAB-rich mixed micelles formed by the cationic surfactant hexadecyl trimethyl ammonium bromide (CTAB) and the anionic surfactant sodium octyl sulphate (SOS) has been investigated with small-angle neutron scattering (SANS). Small tablet-shaped micelles formed by CTAB are found to grow only weakly in size with increasing surfactant concentration. The extent of growth becomes increasingly stronger as the fraction of SOS is increased. At higher fractions of SOS, a rather weak growth at low surfactant concentrations is followed by a sharp increase in aggregation numbers beyond a certain surfactant concentration. Such an abrupt transition from weakly to strongly growing micelles has been observed in the past for several micellar systems and is usually referred to as the second critical micelle concentration. The growth behaviour has been rationalized from a theoretical point of view by means of employing the recently developed general micelle model. The theory excellently predicts micellar growth behaviours as well as the observed correlation between the geometrical shape and micellar growth. In accordance, both width and length are found to slightly increase for weakly growing tablet-shaped micelles. On the other hand, strongly growing micelles that are observed above the second cmc display a completely different behaviour, according to which the length increases considerably while the width of the micelles decreases. Most interestingly, by means of optimizing the agreement between the general micelle model and experimentally determined aggregation numbers, we are able to determine the three bending elasticity constants: spontaneous curvature, bending rigidity and saddle-splay constant.

 Please wait while we load your content...
Please wait while we load your content...