Solution scattering studies of the hierarchical assembly of porphyrin trimers based on benzene triscarboxamide†
Abstract
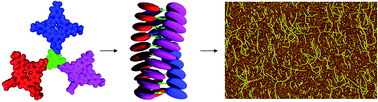
The self-assembly of achiral and chiral porphyrin trimers based on benzene triscarboxamide in solution is studied with the help of NMR, FT-IR, UV-vis, and CD spectroscopy. These studies revealed that in apolar solvents the porphyrin trimers self-assembled in columnar stacks via a combination of hydrogen bonding and π–π stacking interactions. While the critical aggregation constant is about 0.2 mM in chloroform, aggregation already occurs at micromolar concentrations in n-hexane. Small angle neutron scattering (SANS) studies in chloroform, toluene, and n-hexane confirmed aggregation of the trimers into columnar stacks. In chloroform and n-hexane, but not in toluene, the trimers gelated the solvent. In chloroform the stacks of the achiral trimer were found to contain on average about 70 molecules, while in toluene the stacks were much smaller and contained on average 7–9 molecules. In n-hexane the SANS studies revealed that the chiral trimer formed a gel with an average mesh size of the transient network of chains of approximately 90 nm, with chains being built up from effective cylindrical aggregates with an average length of 20 nm.

 Please wait while we load your content...
Please wait while we load your content...