Quenched microemulsions: a new route to proton conductors
Abstract
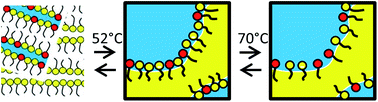
Solid-state proton conductors operating under mild temperature conditions (T < 150 °C) would promote the use of electrochemical devices as fuel cells. Alternatives to the water-sensitive membranes made of perfluorinated sulfonated polymers require the use of protogenic moieties bearing phosphates/phosphonates or imidazole groups. Here, we formulate microemulsions using water, a cationic surfactant (cetyltrimethyl ammonium bromide, CTAB) and a fatty acid (myristic acid, MA). The fatty acid acts both as an oil phase above its melting point (52 °C) and as a protogenic moiety. We demonstrate that the mixed MA–CTA film presents significant proton conductivity. Furthermore, bicontinuous microemulsions are found in the water–CTAB–MA phase diagram above 52 °C, where molten MA plays both the role of the oil phase and the co-surfactant. This indicates that the hydrogen-bond rich MA–CTA film can be formulated in the molten phase. The microemulsion converts into a lamellar phase upon solidification at room temperature. Our results demonstrate the potential of such self-assembled materials for the design of bulk proton conductors, but also highlight the necessity to control the evolution of the nanostructure upon solidification of the oil phase.

 Please wait while we load your content...
Please wait while we load your content...