Prediction of the structure of a silk-like protein in oligomeric states using explicit and implicit solvent models
Abstract
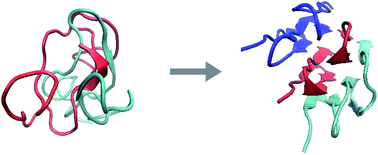
We perform Replica Exchange Molecular Dynamics (REMD) simulations on a silk-like protein design with amino-acid sequence [(Gly-Ala)3-Gly-Glu]5 to investigate the stability of a single protein, a dimer, a trimer and a tetramer made up of these proteins starting from β-roll and β-sheet structures in both explicit (TIP3P) and implicit (GBSA) solvent models. Our simulation results for the implicit solvent model agree with those for the explicit solvent model for simulation times up to the longest tested, being 30 ns per replica. From this we infer that the implicit solvent model that we use is reliable, allowing us to reach much longer time scales (up to 200 ns per replica). We find that the self-assembly of fibers of these proteins in solution must be a nucleated process, involving nuclei made up of at least three monomers. We also find that the conformation of the protein changes upon assembly, i.e., there is a transition from a disordered globular state to an ordered β-sheet structure in the self-assembled state of aggregates containing more than two monomers. This indicates that autosteric effects must be important in the polymerization of this protein, reminiscent of what is observed for β-amyloids. Our findings are consistent with recent experimental results on a protein with an amino acid sequence similar to that of the protein we study.
- This article is part of the themed collection: Silk and silk-inspired materials

 Please wait while we load your content...
Please wait while we load your content...