Novel poly(ethylene glycol) gel electrolytes prepared using self-assembled 1,3:2,4-dibenzylidene-d-sorbitol
Abstract
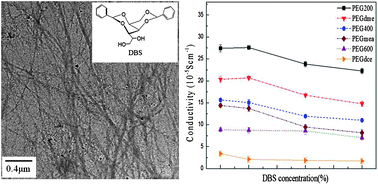
Gel electrolytes have usually been prepared by adding gelators or polymers to the liquid organic solvent-based electrolytes. In this study, we proposed a method to prepare gel electrolytes using gelators in liquid (low molecular weight) polymer-based electrolytes. Inexpensive 1,3:2,4-dibenzylidene-D-sorbitol (DBS) was chosen as a gelator for poly(ethylene glycol) (PEG)-based electrolytes at relatively low DBS concentrations. A series of gel electrolytes was produced by varying the DBS amounts, PEG molecular weights and PEG end groups. First, we found that DBS molecules self-assembled into 3-D networks consisting of nanofibrils that were approximately 10 nm in diameter, as measured by transmission electron microscopy; they exhibited spherulite-like morphologies under polarizing optical microscopy. Second, the dynamic rheological measurements demonstrated that the elastic modulus and the dissolution temperature of DBS–PEG gels increased with the increasing DBS content. The thermal degradation temperature of these gels also increased when the DBS concentration increased, as determined by thermogravimetric analysis. In addition, adding DBS may help to facilitate the dissolution of iodide and iodine in PEG due to its ether groups. Furthermore, the conductivity of the prepared DBS–PEG gel electrolytes was similar to that of the liquid PEG electrolytes (without DBS). When used in dye-sensitized solar cells (DSSC), the PEG-based electrolytes having inactive methyl end groups achieved the highest energy conversion efficiency among the tested cells. The efficiency of DSSC filled with our gel electrolytes remained basically the same over a one-month period, implying that the materials were relatively stable.

 Please wait while we load your content...
Please wait while we load your content...