Conjugated oligomers incorporating azulene building blocks – seven- vs. five-membered ring connectivity†
Abstract
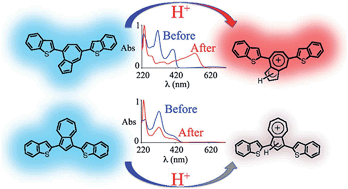
The properties of isomeric azulene derivatives based on 7- versus 5-membered ring substitution were examined by the synthesis and characterization of well-defined electroactive oligomers. The substitution pattern was shown to dramatically influence solid-state, electronic and optical properties of the oligomers with acid-responsive materials only being observed when the azulenium cation could be directly stabilized by substituents in the 7-membered ring. Protonation was accompanied by a reversible color change and a strong red-shift of the absorption maximum as indicated by UV-vis studies. In addition, we show that the absorption maxima and optical band-gaps of azulenium cations can be tuned by the nature of the chromophore connected to the seven-membered ring of the azulene nucleus.

 Please wait while we load your content...
Please wait while we load your content...