Organic super-electron-donors: initiators in transition metal-free haloarene–arene coupling†
Abstract
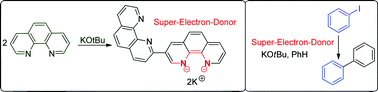
Recent papers report transition metal-free couplings of haloarenes to arenes to form biaryls, triggered by alkali metal tert-butoxides in the presence of various additives. These reactions proceed through radical intermediates, but understanding the origin of the radicals has been problematic. Electron transfer from a complex formed from potassium tert-butoxide with additives, such as phenanthroline, has been suggested to initiate the radical process. However, our computational results encouraged us to search for alternatives. We report that heterocycle-derived organic electron donors achieve the coupling reactions and these donors can form in situ in the above cases. We show that an electron transfer route can operate either with phenanthrolines as additives or using pyridine as solvent, and we propose new heterocyclic structures for the respective electron donors involved in these cases. In the absence of additives, the coupling reactions are still successful, although more sluggish, and in those cases benzynes are proposed to play crucial roles in the initiation process.

 Please wait while we load your content...
Please wait while we load your content...