Formal anti-Markovnikov hydroamination of terminal olefins†
Abstract
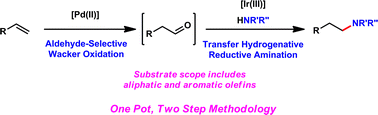
A new strategy to access linear amines from terminal olefin precursors is reported. This two-step, one-pot hydroamination methodology employs sequential oxidation and reduction catalytic cycles. The formal hydroamination transformation proceeds with excellent regioselectivity, and only the anti-Markovnikov product is observed. Up to 70% yield can be obtained from styrenes or aliphatic olefins and either primary or secondary aromatic amines. Additionally, the scope is broad with respect to the olefin and accommodates a variety of functionalities; we demonstrate that amines with removable aryl protecting groups may be utilized to allow access to a more diverse array of hydroamination adducts.

 Please wait while we load your content...
Please wait while we load your content...