Electrostatic control of regioselectivity via ion pairing in a Au(i)-catalyzed rearrangement†
Abstract
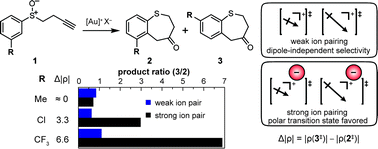
The rearrangement of 3-substituted aryl alkynyl sulfoxides catalyzed by cationic Au(I) complexes was studied with different counterions in solvents spanning a range of dielectric constants (ε). Pulsed-gradient diffusion NMR experiments demonstrated strong ion pairing in low-ε solvents. The regioselectivity of the reaction was insensitive to ε when ion pairing was weak but increased monotonically as ε was decreased in the regime of strong ion pairing. DFT calculations of putative product-determining transition states indicated that the product resulting from the more polar transition state is favored due to electrostatic stabilization in the presence of strong ion pairing.

 Please wait while we load your content...
Please wait while we load your content...