Novel artificial metalloenzymes by in vivo incorporation of metal-binding unnatural amino acids†
Abstract
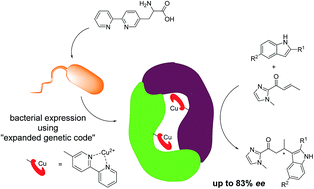
Artificial metalloenzymes have emerged as an attractive new approach to enantioselective catalysis. Herein, we introduce a novel strategy for preparation of artificial metalloenzymes utilizing amber stop codon suppression methodology for the in vivo incorporation of metal-binding unnatural amino acids. The resulting artificial metalloenzymes were applied in catalytic asymmetric Friedel–Crafts alkylation reactions and up to 83% ee for the product was achieved.

 Please wait while we load your content...
Please wait while we load your content...