Elucidating the relationship between substrate and inhibitor binding to the active sites of tetrameric β-galactosidase†
Abstract
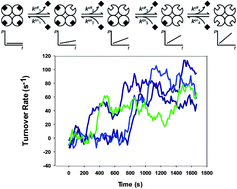
The activities of hundreds of single molecules of β-galactosidase were monitored in the presence of fluorogenic substrates and two strong binding inhibitors—D-galactal and N-p-bromobenzylamino-hydroxymethyl-cyclopentanetriol (NpBHC). The stochastic binding and release of the inhibitors to single β-galactosidase molecules was studied in both pre-steady state and steady state conditions. The effect of inhibition on enzyme activity is described and compared for both inhibitors. The inhibitor exchange rate and the substrate turnover rate were computed for individual enzyme molecules. These parameters are shown to be heterogeneous across the enzyme population. We demonstrate an inverse correlation between these parameters thus demonstrating that competitive inhibition is tightly coupled to the nature of the active site of individual enzyme molecules.

 Please wait while we load your content...
Please wait while we load your content...