Small molecule probes that perturb a protein–protein interface in antithrombin†
Abstract
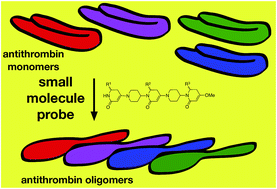
Small molecule probes for perturbing protein–protein interactions (PPIs) in vitro can be useful if they cause the target proteins to undergo biomedically relevant changes to their tertiary and quaternary structures. Application of the Exploring Key Orientations (EKO) strategy (J. Am. Chem. Soc., 2013, 135, 167–173) to a piperidinone–piperidine chemotype 1 indicated specific derivatives were candidates to perturb a protein–protein interface in the α-antithrombin dimer; those particular derivatives of 1 were prepared and tested. In the event, most of them significantly accelerated oligomerization of monomeric α-antithrombin, which is metastable in its monomeric state. This assertion is supported by data from gel electrophoresis (non-denaturing PAGE; throughout) and probe-induced loss of α-antithrombin's inhibitor activity in a reaction catalyzed by thrombin. Kinetics of α-antithrombin oligomerization induced by the target compounds were examined. It was found that probes with O-benzyl-protected serine side-chains are the most active catalysts in the series, and reasons for this, based on modeling experiments, are proposed. Overall, this study reveals one of the first examples of small molecules designed to act at a protein–protein interface relevant to oligomerization of a serpin (i.e. α-antithrombin). The relevance of this to formation of oligomeric serpin fibrils, associated with the disease states known as “serpinopathies”, is discussed.

 Please wait while we load your content...
Please wait while we load your content...