Trapping of the putative 1,2-dinitrosoarene intermediate of benzofuroxan tautomerization by coordination at ruthenium and exploration of its redox non-innocence†
Abstract
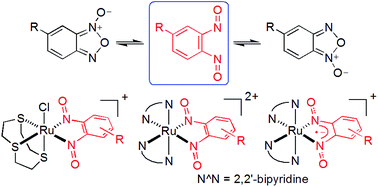
The reaction of a benzofuroxan with [Ru([9]aneS3)(dmso)Cl2] ([9]aneS3 = 1,4,7-trithiacyclononane) and [Ru(bpy)2(CH3CN)2]2+ yields the ruthenium complexes [Ru([9]aneS3)(ON^NO)(Cl)]+ (1a–c) and [Ru(bpy)2(ON^NO)]n+ (n = 2: 2a and 2b; n = 1: 2a− and 2b−), respectively, containing neutral or monoanionic N,N′-coordinated 1,2-dinitrosoarenes (ON^NO). The oxidation states of the ON^NO ligands (0 for 1a–c, 2a and 2b; −1 for 2a− and 2b−) have been deduced through detailed structural, spectroscopic, and theoretical studies. In other words, not only does this work demonstrate the trapping of the putative 1,2-dinitrosoarene intermediate of benzofuroxan tautomerization by coordination to ruthenium, it also provides access to a new family of redox-active bidentate ligands.

 Please wait while we load your content...
Please wait while we load your content...