Synthesis and chemoselective ligations of MIDA acylboronates with O-Me hydroxylamines†
Abstract
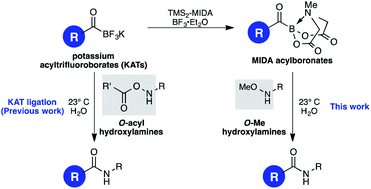
N-Methyliminodiacetyl (MIDA) acylboronates undergo chemoselective amide-bond forming ligations in water with O-Me hydroxylamines, including unprotected peptide substrates. These bench-stable boronates were easily prepared from potassium acyltrifluoroborates (KATs) in one step. The reactivity of MIDA acylboronates with O-alkylhydroxylamines – which are unreactive with KATs – was attributed to the nature of the neutral MIDA boronates versus the ionic KATs, leading to differences in the stability of likely intermediates and propensity for elimination.

 Please wait while we load your content...
Please wait while we load your content...