Fluorescent and chemico-fluorescent responsive polymers from dithiomaleimide and dibromomaleimide functional monomers†
Abstract
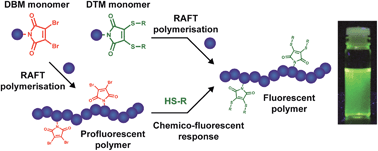
A new class of brightly fluorescent and profluorescent methacrylate and acrylate monomers is reported. The fluorescent monomers contain the dithiomaleimide (DTM) fluorophore, which imparts a large Stokes shift (up to 250 nm) and bright emission. Furthermore, the simple and efficient chemistry of the DTM group, as well as its excellent processability (highly soluble, neutral functional group) makes monomer preparation straightforward. Copolymerisation at 10 mol% loading with a range of hydrophobic and hydrophilic monomers is demonstrated by RAFT polymerisation. Reactions proceed to high monomer conversion with excellent control over molecular weight (ĐM < 1.3) under standard polymerisation conditions. Incorporation of these fluorescent DTM-functional monomers has little effect on polymer properties, with PEG (meth)acrylate copolymers retaining their water solubility and thermoresponsive behaviour. A thiol-exchange reaction is also possible, whereby the thiol ligands of the pendent DTM groups can be exchanged by conjugate addition–elimination with an alternative thiol. Monomers containing the dibromomaleimide (DBM) group gave profluorescent copolymers. Reaction of the DBM group with thiols (to form the DTM group) corresponds to a chemico-fluorescent response, leading to an OFF-to-ON switching of fluorescence. This post-polymerisation functionalisation is shown to be fast and highly efficient (>95% conversion in 3 h), and by using thiols of different polarities can be used to progressively tune the LCST cloud point of a thermoresponsive polymer over a range of 11 °C. Therefore, both DTM and DBM functional monomers provide a simple and effective tool for fluorescent labelling of (meth)acrylate polymers.

 Please wait while we load your content...
Please wait while we load your content...