Convergent, asymmetric synthesis of vicinal amino alcohols via Rh-catalyzed addition of α-amido trifluoroborates to carbonyls†
Abstract
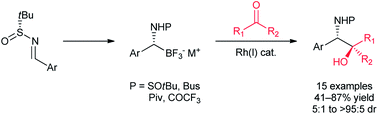
We describe the Rh-catalyzed addition of α-sulfinamido trifluoroborates to carbonyl compounds for the convergent, asymmetric synthesis of vicinal amino alcohols. This method represents the first application of α-amino boron reagents as reaction partners in rhodium-catalyzed couplings. Reactions with trifluoromethyl ketones proceed in reasonable yields and with good diastereoselectivity along with complete retention at the organoboron stereocenter. The potential of this method is further highlighted by the exploration of a variety of nitrogen substituents and addition to benzaldehyde and trityl-protected isatin.

 Please wait while we load your content...
Please wait while we load your content...