Enantioenriched β-lactone and aldol-type products from regiodivergent carbonylation of racemic cis-epoxides†
Abstract
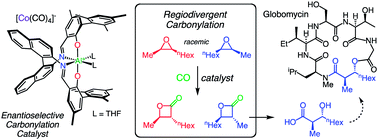
A new carbonylation catalyst is reported that provides enantioenriched β-lactones and aldol-type products from the carbonylative ring-expansion of racemic cis-epoxides. Detailed analysis of the reaction demonstrates that the epoxide substrates undergo regiodivergent carbonylation reactions instead of traditional kinetic resolutions. This new catalytic system was applied to the synthesis of a key fragment of the antibiotic Globomycin.

 Please wait while we load your content...
Please wait while we load your content...