Single-conformation UV and IR spectroscopy of model G-type lignin dilignols: the β–O–4 and β–β linkages†
Abstract
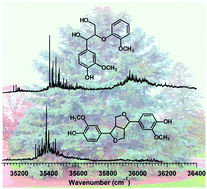
Single-conformation ultraviolet and infrared spectroscopy was performed on dilignols containing two of the three biologically prevalent β-lignol linkages, erythro β–O–4 (β-aryl ether) and (±) β–β (pinoresinol). Both dilignols contain guaiacol(G)-type sub-units, representative of these linkages in G-type lignin. Resonant two-photon ionization (R2PI), IR-UV, and UV-UV holeburning (UVHB) spectroscopy in the cold, isolated environment of a supersonic expansion was carried out to determine the spectroscopic signatures associated with each linkage conformation, revealing striking differences in the vibronic intensity patterns between the two molecules in the UV. Two conformational isomers were found for the β–O–4 dilignol, both being classified into the fully hydrogen-bonded family with α-OH⋯OCH3 (C8) and γ-OH⋯Oβ (C5) H-bonds that are characteristic of the β–O–4 linkage. Conversely, a single dominant conformation was found for the conformationally-constrained pinoresinol. Resonant ion-dip infrared (RIDIR) spectroscopy provided conformation-specific IR spectra in the OH stretch and alkyl CH stretch regions, yielding complementary data that reported on both the intramolecular H-bonding and more subtle linkage features, respectively. DFT M05-2X calculations predict that the rigid β–β linkage had far fewer low-energy conformations in the first 20 kJ mol−1 (3) than the more flexible β–O–4 linkage (45). In the β–O–4 lignin dimer, the distinct UV chromophores lead to a splitting between S1 and S2 states that is determined mainly by the differences in the chemical structures of the two chromophores. In pinoresinol however, the assigned structure has C2 symmetry, with a calculated vertical excitonic splitting between the S1 and S2 states of 74 cm−1 (TDDFT). After taking into account the reduction in splitting associated with the geometry change in the aromatic rings upon electronic excitation, a vibronically quenched excitonic splitting of no more than a few wavenumbers is predicted for the C2 symmetric pinoresinol, but a definite experimental confirmation was not possible. These results predict that, under most circumstances, adjacent chromophores along a lignin polymer chain are not significantly electronically coupled to one another, and can be treated largely as isolated chromophores.

 Please wait while we load your content...
Please wait while we load your content...