Asymmetric synthesis of chiral cycloalkenone derivatives via palladium catalysis†
Abstract
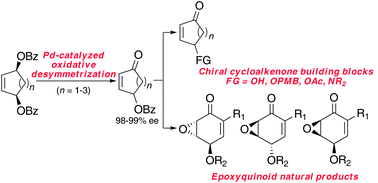
The palladium-catalyzed oxidative desymmetrization of meso-dibenzoates yields γ-benzoyloxy cycloalkenones in good yields and with excellent levels of enantioselectivity. These compounds serve as precursors to a broad range of substituted cycloalkenones, including well-established synthetic building blocks and elaborated cycloalkanone derivatives. The ability to prepare both enantiomers of the oxidative desymmetrization products enables a unified strategy toward stereochemically diverse epoxyquinoid natural products.

 Please wait while we load your content...
Please wait while we load your content...