Total synthesis and characterization of thielocin B1 as a protein–protein interaction inhibitor of PAC3 homodimer†
Abstract
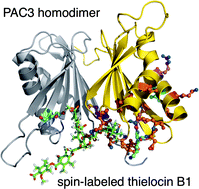
We have characterized the inhibition of the protein–protein interaction of the homodimer of proteasome assembling chaperone (PAC) 3 with thielocin B1, discovered from natural product sources. Molecular modeling using docking studies and molecular dynamics simulation suggested that thielocin B1 exhibits distinct binding positions on the interface of the homodimer and the complex can be stabilized by five interactive residues on PAC3. Thielocin B1 was synthesized for the first time and was utilized for nuclear magnetic resonance (NMR) titration of the PAC3 homodimer. The data revealed significant chemical shift perturbations observed in eight residues on PAC3. We also synthesized a spin-labeled derivative to observe paramagnetic relaxation enhancement (PRE) effects. As a result, distinct decrease of the intensities of the NH peaks was observed in sixteen residues of PAC3 in the presence of the spin-labeled derivative. Both the NMR experiments and further in silico docking studies have suggested that thielocin B1 approaches one face of the PAC3 homodimer, not the monomer, releasing the subunit of the interface of the PAC3 homodimer by a rare pre-dissociation-independent mechanism.

 Please wait while we load your content...
Please wait while we load your content...