Insitu thioester formation for protein ligation using α-methylcysteine†
Abstract
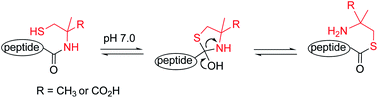
The progress of total chemical protein synthesis has been hampered by difficulties in preparing peptide thioesters by standard Fmoc peptide synthesis. The amino acid, α-methylcysteine, sited at the C-terminus of a peptide can substitute for a thioester in peptide ligation reactions. C-terminal α-methylcysteine is fully compatible with Fmoc peptide synthesis and its use in ligation is very simple and robust. Its potential is demonstrated with the synthesis of model proteins.

 Please wait while we load your content...
Please wait while we load your content...